

3 hydrogen atoms are bonded to oxygen, so the number of the monovalent atoms (M) 3 As this is a cationic molecule thus, C 1.
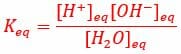
Methylamine Methylamine IUPAC name aminomethane Other names monomethylamineMMA Methylamine is the chemical compound with a formula of CH3NH2. The results can reveal the influences of different ions/groups on ORR catalytic mechanisms of GQDs and provide guidance for the design of ORR electrocatalysts. In hydronium ion, the central atom is oxygen and it has 6 valence electrons. :, , Methylamine, Methanamine, Aminomethane, Monomethylamine. Analyzing differential electron density illustrates that when H 3O is adsorbed to gN-GQDs, the adsorption electron transfer of O 2 is more obvious to facilitate the adsorption of O 2. The findings indicate that the adsorption of H 3O on the surface of gN-GQDs has the best ORR catalytic performance and can effectively promote oxygen reduction reactions. The ORR overpotentials of gN-GQDs with H 3O +, OH –, H 3O and OH are 1.49, 1.80, 0.69, and 0.83 V, respectively. Chemistry and Chemical Reactivity, Volume 1 (7th Edition) Edit edition Solutions for Chapter 9 Problem 1E: Valence Bond Description of BondingUse valence bond theory to describe the bonding in the hydronium ion, H3O+, and methylamine, CH3NH2. The electrocatalytic activities of different ions/groups on GQDs are different for the presence of ions/groups can rearrange the electronic structure of doped GQDs. The ORR catalytic activity can be enhanced by ligand functionalization of graphitic N-doped GQDs (gN-GQDs) with H 3O and OH. All the hydrogens attached to the oxygen are exactly equivalent in the coordinate bond formed in the hydronium ion. In this work, density functional theory (DFT) is used to investigate the effects of different forms of H 3O +, OH –, H 3O and OH on oxygen reduction reaction (ORR) electrocatalytic properties of graphene quantum dots (GQDs). The crystal packing is mainly directed by strong hydrogen bonding interactions observed between the hydronium ion and NiL complexes but, in addition, weak interactions such as and CH are also observed. The correct option is C Coordinate bond A co-ordinate bond (also called a dative covalent bond) is a covalent bond in which both electrons come from the same atom.


 0 kommentar(er)
0 kommentar(er)
